Our Purpose
Take advanced medical devices and technology to physicians teams all over Brazil. We work with imported products and equipments to assure accurate diagnoses and effective procedures.
know E. Tamussino Ethics and Compliance-
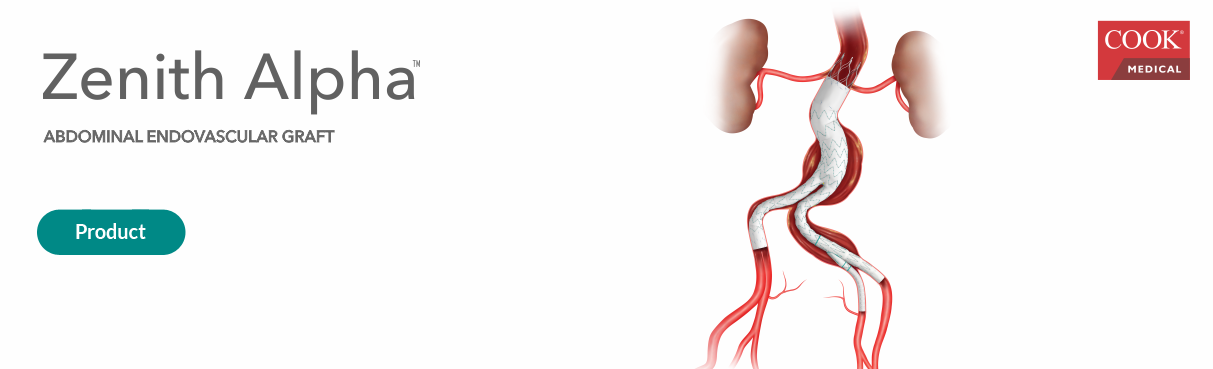
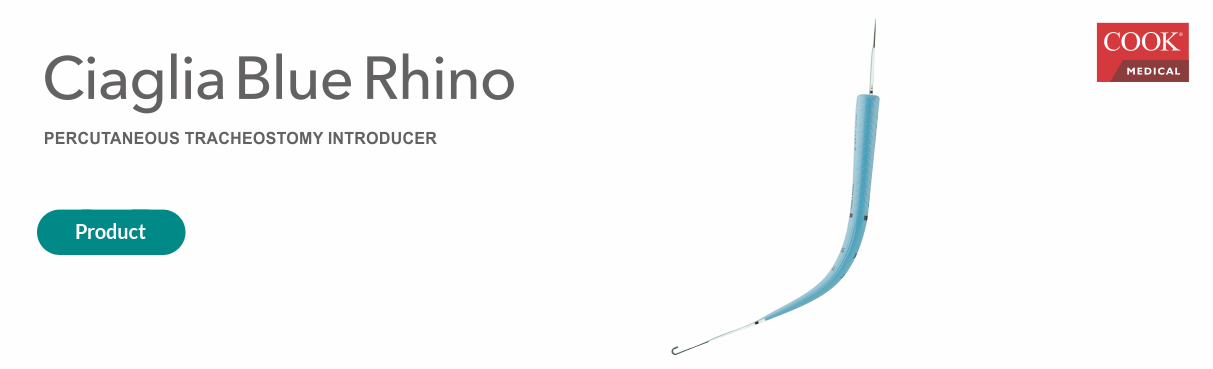
Founded in 1963 in Bloomington, Indiana, CookMedical is a company present in more than 135 countries that provides the types of products in the areas of interventional radiology, vascular, endoscopy, intentional therapy, among others.
-
ERBE is a German company founded in 1851 that develops and manufactures surgical systems for electrosurgery, vessel sealing, argon plasma coagulation, cryosurgery and hydro surgery. Erbe is considered a pioneer in electrosurgery, a technology that uses high frequency currents to cut, coagulate or devitalize tissues, or to seal blood vessels.
-
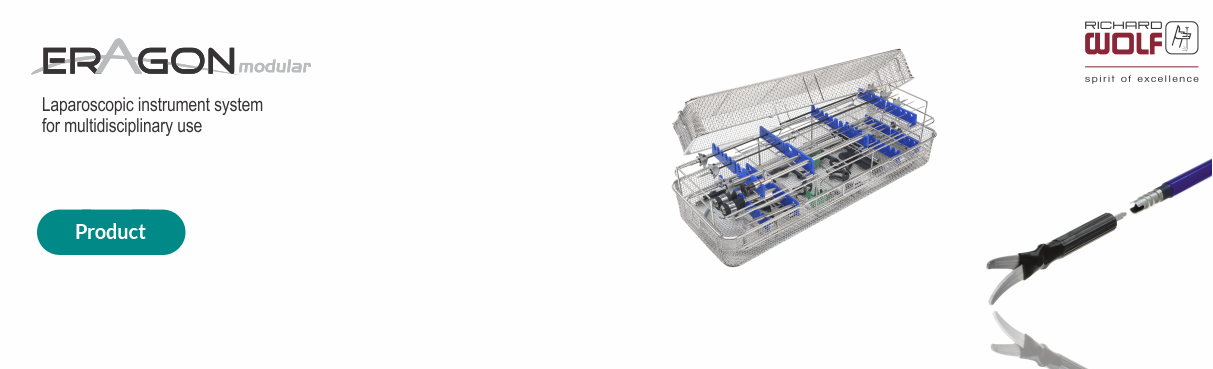
Richard Wolf was founded in 1906 in Berlin with the aim of offering a wide range of products and systems for endoscopy and treatment of extracorporeal shockwaves. The company has a wide range of imaging products suitable for Laparoscopy, Urology and Gynecology, among others.
Your life matters
Our priority is to take care of lifes! To this end, we work to assure medical devices and equipment wich enable an accurate diagnosis, less traumatic procedures to a better quality of life.
More about us